Contact Us
Research
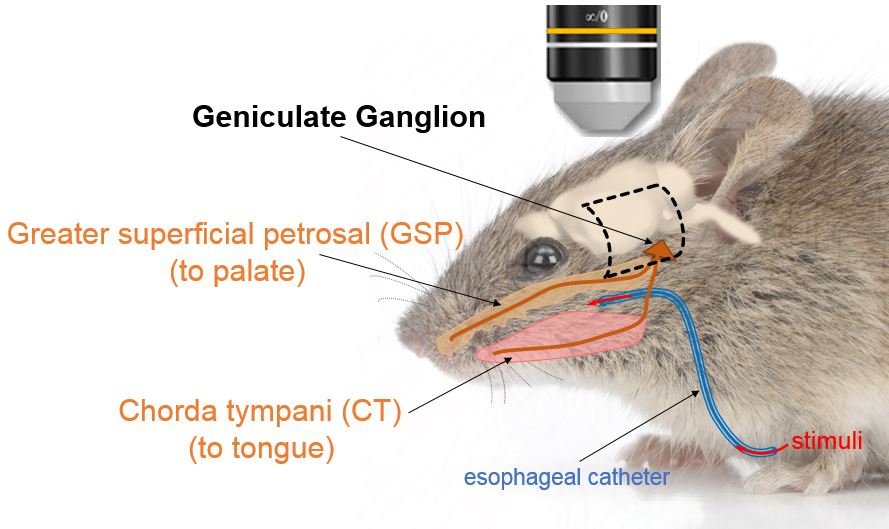
Our laboratory studies sensory neurobiology, and specifically the transmission of orosensations (e.g., taste, pain, temperature, irritating chemicals) from peripheral sensory structures into the brain.
Ongoing projects include tests of orofacial pain and taste dysfunction that result from off target side effects of cancer chemotherapy such as oxaliplatin- and cisplatin-induced oral cold allodynia and dysgeusia. The premise is that taste changes and orofacial pain after oxaliplatin or cisplatin chemotherapy may be due, in part, to pathological changes in the sensory ganglia that innervate the tongue, head, and neck--the geniculate and trigeminal ganglia. Because these ganglia lie outside the blood-brain barrier, pathological changes there may be treatable by drugs and agents injected into the blood stream, raising hopes for developing effective means to alleviate the painful side effects of chemotherapy.
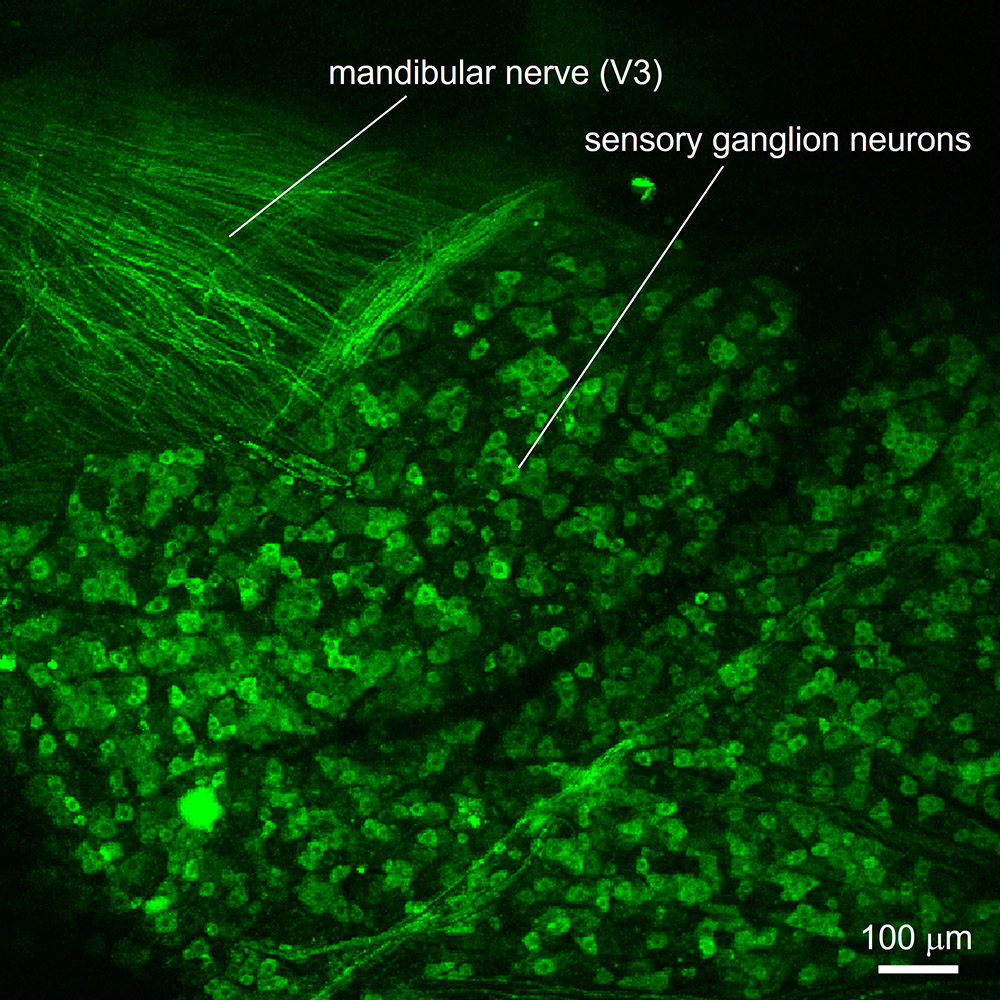
In a parallel project, we are investigating the cellular and molecular mechanisms underlying oral cancer pain. Oral cancer is intensely painful. There are few treatments apart from opioids and surgical resection. We are using sophisticated in vivo laser scanning confocal calcium imaging to measure nociceptor activity in a new mouse model for oral cancer. Specifically, we use mice that express the calcium-sensitive protein, GCaMP, in sensory afferent neurons. This allows us to image the activity of these sensory neurons in a living, anesthetized animal where the trigeminal ganglion has been surgically exposed. Neuronal activity is imaged using a scanning laser confocal microscope equipped with a long-working distance, high-power objective.
![Neuronal activity (Ca2+ responses) in trigeminal ganglion neurons recorded in vivo, and evoked by stimulating the oral cavity with common spices (mustard oil [AITC], menthol, capsaicin) and cold temperature.](/-/media/sylvester-comprehensive-cancer-center/labs/roper-lab/inline-images/joijourlab2.ashx?h=334&w=561&rev=9491cd3e004346b485598ccbcc0163aa&hash=5B725E3AD2342ABACBF80EE0CB61764A)
Last, we are conducting a series of studies focusing on oral chemosensory receptor mechanisms and molecular/functional connectivity in the taste pathway. Specifically In particular, ongoing projects involve in vivo confocal Ca2+ imaging of taste-evoked responses in sensory neurons in the geniculate ganglion; molecular-functional definition of gustatory neurons and pathways; and/or transgenic- and viral-tracing to establish gustatory circuitry by cell type.