Ensuring high quality, safe cancer care during the pandemic
In 2020, the rapid emergence of COVID-19 tested major cancer centers across the U.S. in our ability to quickly change course, collect and share data, remain highly responsive to vulnerable patients’ needs, and rally to advance cancer research despite a raging pandemic. Sylvester not only passed those tests, but also led the nation with innovative approaches to the unique challenges brought on by a novel and dangerous virus.
Gilberto Lopes, M.D., associate director of global oncology at Sylvester Comprehensive Cancer Center, was among a small group of oncologists harnessed social media’s power and started a data registry to understand how COVID-19 was impacting cancer patients. The COVID-19 and Cancer Consortium was launched in March and Sylvester was one of five founding institutions.

By May 2020, the Consortium presented 30-day all-cause mortality data on 928 cancer patients with confirmed SARS CoV-2 at the annual meeting of the American Society of Clinical Oncology (ASCO). The Lancet published the group’s findings May 28, 2020. The consortium’s groundbreaking study helped to provide clarity when there was none on COVID-19 and cancer.
 New laboratory established on the medical campus to process COVID-19 tests.
New laboratory established on the medical campus to process COVID-19 tests.Sylvester is located in an area of South Florida that has been a COVID-19 hotspot throughout the pandemic. Miami Dade-County leaders turned to Sylvester researchers when they were looking to quantify the extent of COVID-19 infection in the county.
Sylvester Firefighter Cancer Initiative Director Erin Kobetz, Ph.D., M.P.H., and Deputy Director Alberto Caban-Martinez, D.O., Ph.D., M.P.H., worked in March 2020 to begin assessing infection rates in the general population and among first responders, using rapid antibody testing. The Surveillance Program Assessing Risk and Knowledge of Coronavirus (SPARK-C) was the first initiative of its kind in the nation.
Stephen D. Nimer, M.D., director of the Sylvester Comprehensive Cancer Center, was charged with developing COVID testing and algorithms for patients and employees of the Miller School of Medicine and the entire University of Miami Health System.
In three weeks, a new laboratory was set up in the medical school campus with borrowed instruments from school researchers and started offering hundreds of COVID-19 tests daily for patients and health care workers. That expanded to include students and staff at the University of Miami.
“When we began, we were in crisis mode,” Dr. Nimer said. “We had an unfamiliar disease with no playbook, no established testing programs, no protocols. Yet we went from zero testing capacity to 500 tests a day in a few weeks. Now we can do 2,000 tests a day and know the answer in 30 minutes, four hours, 24 hours or 48 hours—depending on the circumstances that the test is ordered.” By the end of the year, the molecular pathology lab had performed more than 130,000 tests.
As South Florida’s only National Cancer Institute (NCI)-designated cancer center with one of the largest and busiest clinical trial programs in the area, Sylvester continued to provide investigational therapies during the pandemic, while ensuring patient safety. When possible oral drugs or other self-administered therapies were shipped to patient homes, so they did not have to physically pick up their investigational medications.
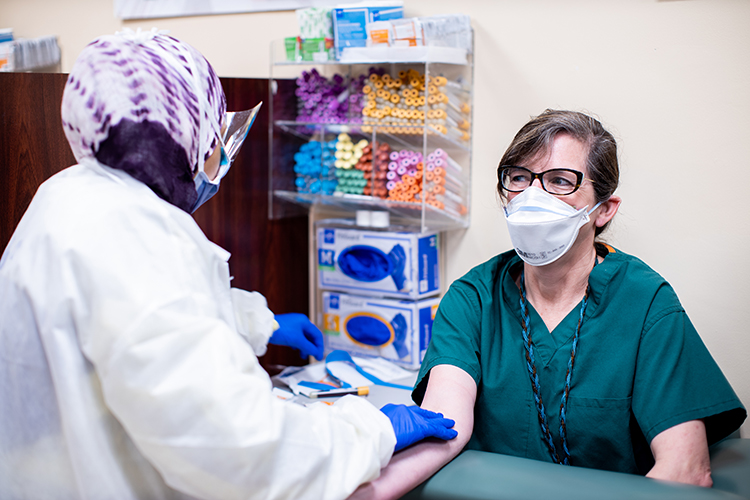 Antibody testing of medical staff at Sylvester
Antibody testing of medical staff at SylvesterThe clinical research team and Sylvester staff limit patient exposure to COVID-19 by conducting routine follow-up appointments using telehealth. Physicians have learned to use telemedicine effectively, despite not having the option of human touch.
“Since I cannot place a reassuring hand on their shoulder nor give them a hug, I have placed more emphasis on making eye contact and choosing the right words at the right time,” said Jonathan Trent, M.D., Ph.D., professor of medicine and associate director of clinical research.
When patients with cancer require in-person care, and a no-visitor policy in place for safety, Sylvester nursing team members make sure those patients are not alone, often sitting with them to provide company and comfort before patients get lab results, therapy or during treatment.
Sylvester’s 40-bed in-patient facility, used primarily for stem cell transplant patients, CAR T-cell patients and leukemia patients, does not house COVID-19 patients. As a result, Sylvester continued to provide stem cell transplants and CAR T-cell therapy.
At the start of the pandemic some surgeries needed to be postponed, but urgent life-saving procedures continued. In mid-April Ricardo J. Komotar, M.D., director of the Sylvester’s Brain Tumor Initiative, removed a patient’s 5.5 cm brain tumor using COVID-19 safety precautions. The patient went home the day after his surgery. Since then, carefully selected brain tumor procedures have been performed on an outpatient basis.
In the midst of a pandemic, people with cancer continued to need timely, quality, and safe treatment. And Sylvester was there to provide it. In fact, chemotherapy and radiation therapy volumes were up roughly 10% in 2020 according to Dr. Nimer.