Contact Us
Research
My laboratory's research focus is on developing imaging analysis techniques in translational cancer research, encompassing early detection, diagnosis, and treatment. In this process, we integrate analytical and deep learning methods for the analysis, mining, and interpretation of "big data" generated by high-throughput approaches such as genomics, proteomics, metabolomics, and radiomics.
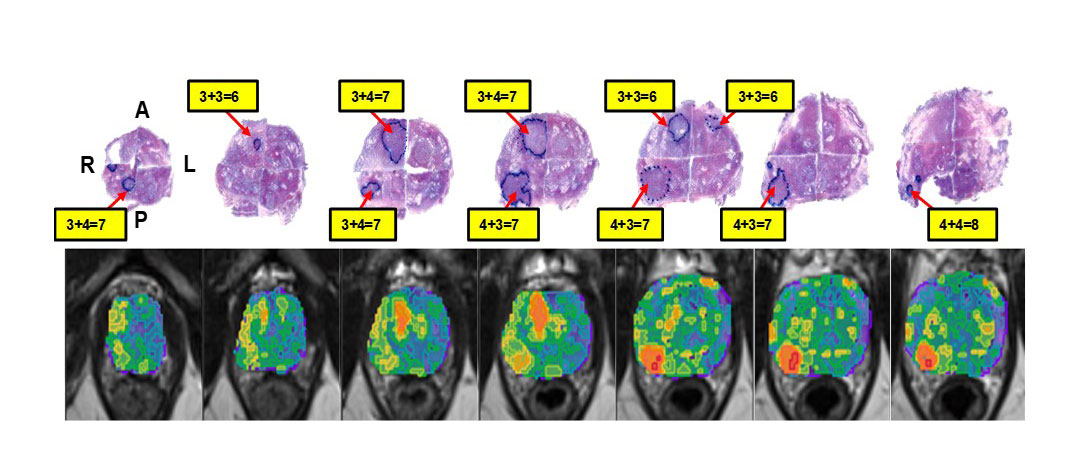
My team has developed software for the analysis of multiparametric (mp)MRI to define habitats of the prostate that carry an increased risk of harboring cancer. The resulting "Habitat Risk Score" (HRS) is a ten-scale system that, on a pixel-by-pixel basis, creates a heatmap with increasing levels associated with a higher risk of the Gleason score. HRS is implemented in an MRI/Ultrasound-fussion platform to guide prostate biopsies and target the most aggressive habitat. The software is now being prospectively evaluated in an NIH-funded clinical trial.
Improving outcomes from radiation therapy is another research focus of my group. Precisely delivering the dose to malignant tissue increases the likelihood of tumor eradication by maximizing the response while minimizing the toxicity of surrounding normal tissue. HRS is also integrated into the radiation treatment workflow by delineating areas in the prostate for radiation boost delivery.
Similar to other 'omics' approaches, "radiomics" employs high-throughput methods to capture quantitative imaging information. Connecting radiomics data from the prostate biopsy location with the genomic expression of the tissue, termed "radiogenomics," plays a central role in my research. Recently, we have adopted deep learning techniques for digital pathology with the goal of linking imaging with tissue characteristics.
The overarching goal is to non-invasively obtain an in vivo map of tumor habitats and heterogeneity. By discovering relationships between gene/biomarker expression/digital pathology and quantitative imaging features, a detailed picture of tumor components (necrosis, viable tissue, vasculature) and the microenvironment (hypoxia) will emerge. Our long-term goal is to integrate this information into mathematical models for tumor progression, allowing us to explore the effects of different treatments in silico. These efforts will guide the design of optimal therapies on a patient-specific, personalized basis.