Researchers at Sylvester have identified an innovative approach for targeting diffuse large B-cell lymphoma (DLBCL) with a front-line chemotherapy drug. Using nanoparticles developed by the University of Miami Department of Chemistry, the cancer researchers were able to deliver doxorubicin (Dox) directly to tumor cells in a laboratory setting while minimizing side effects to other tissues.
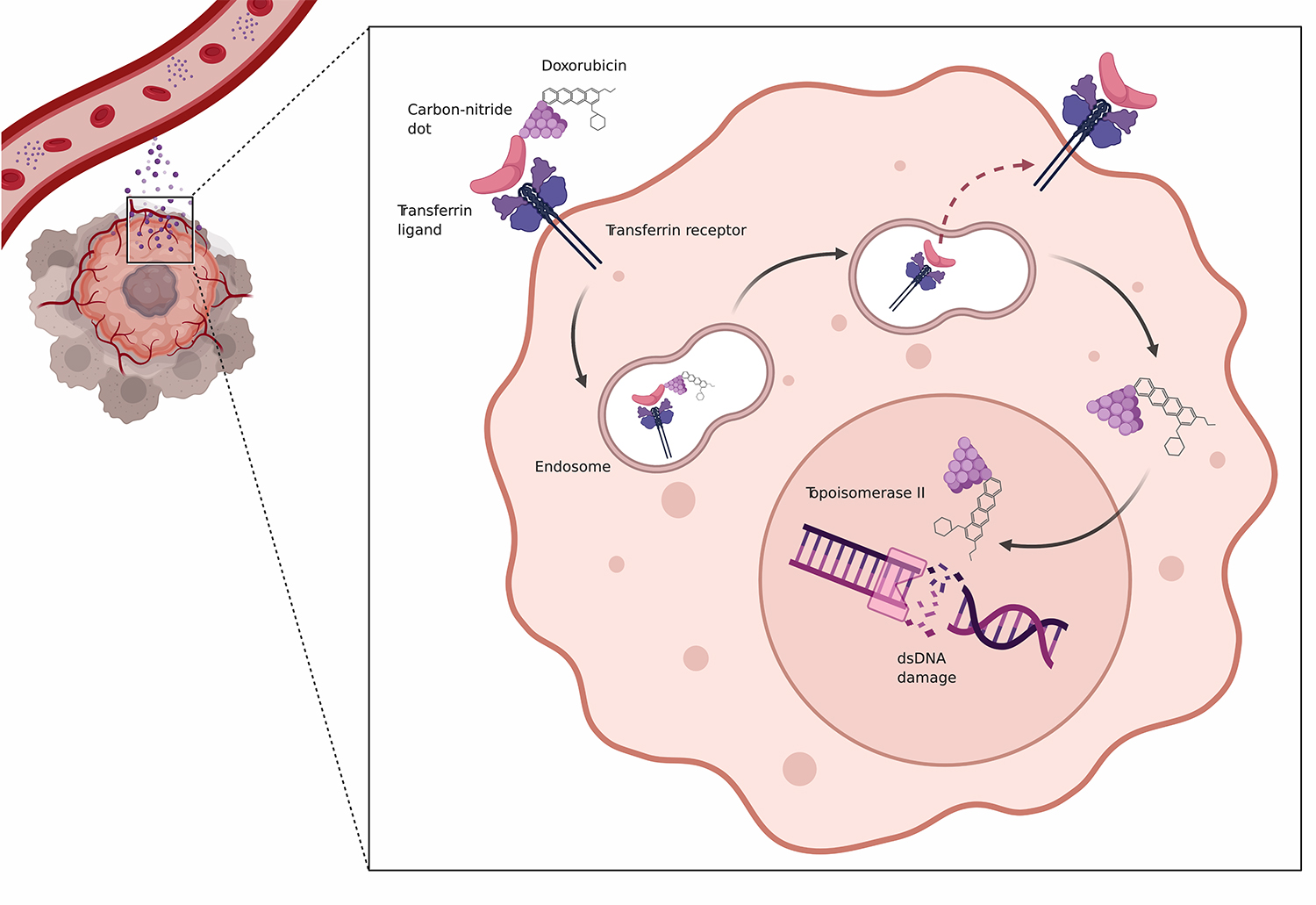
“We are seeking to optimize this powerful drug by blocking the pathways within lymphoma cells that drive their growth and can cause them to become resistant to treatment,” said Jonathan H. Schatz, M.D., associate professor of medicine. “Our recent work focuses on the carrier protein for transferrin, which is needed to bring iron into the cell but can be over-expressed in lymphomas.”

Dr. Schatz is the senior author of a new study, “Optimized Doxorubicin Chemotherapy for Diffuse Large B-Cell Lymphoma Exploits Nanocarrier Delivery to Transferrin Receptors,” published in the journal Cancer Research. The study draws on the work of Roger M. Leblanc, Ph.D., professor and chair of the Department of Chemistry, whose laboratory developed novel nanocarriers called carbon-nitride dots (CND) that can deliver varied molecular compounds to target cells.
Currently, Dox forms the backbone of a five-drug combination treatment called R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) that cures DLBCL – the most common lymphoid malignancy in the United States – more than 60 percent of the time. But because of its toxicity, R-CHOP can only be used for one course of treatment, most commonly six treatment cycles, in any patient, and oncologists must rely on other medications for patients who may not be able to tolerate R-CHOP, do not respond to this treatment, or have a relapse of disease.
In the new study, the CND nanoparticles carrying Dox were effective in honing in on the transferrin receptors on human lymphomas implanted in mouse models, said Dr. Schatz. “We had very encouraging results in a head-to-head comparison with standard therapy,” he added.
In fact, the researchers were able to deliver an additional two cycles of Dox therapy to the mice using the CND delivery approach (R-nanoCHOP), said Artavazd Arumov, a doctoral candidate in cancer biology who led pre-clinical studies and managed the experimental models. “This study provides a proof of principle of our approach for transforming Dox into a more targeted therapy that could be safer with lower toxicity for patients in the future.”
Dr. Schatz said the Sylvester team is looking at making modifications to the CND particles to improve their effectiveness and identify an optimized dose of the chemotherapy drug. “Nanocarrier-mediated Dox delivery could be a potential new therapeutic option in DLBCL,” he said. “We want to make sure this therapeutic is truly safe to administer to humans one day.”